
Introduction to the Enferplex Bovine TB Antibody Test
The Enferplex test is a serological assay (identifies the presence of antibody) for bovine Tuberculosis (bTB).
The test detects the presence of antibodies within blood or milk to bTB by use of individual antigens. There are eleven different antigens used in the test and these are placed separately on individual spots within a testing well. If antibody to bTB is present in the blood sample being tested, then it will bind with the relevant antigen and the resultant reaction produces a luminescent reaction, the light from which can be measured and quantified (see figure 1 and table 1 below).
Figure 1: Images from three individual testing wells showing individual antigen spots that have bound antibody and are luminescent. This light is measured to produce a quantitative result as in Table 1.

Thresholds are set for each individual antigen spot and if the level of Relative Light Units (RLU) as shown in Table 1 is above this threshold, a positive reaction is deemed to have occurred. The relative sensitivity and specificity of the test can be altered by changing the thresholds of the number of individual antigens, and the amount of antibody detected by those individual antigens, that are required to be positive for an animal to be determined as being positive to tuberculosis. The more individual antigens that are required to be positive, and the more antibody that is needed to be detected, for an animal to be deemed positive, then the more specific and the less sensitive the result will be. For the purposes of our application for WOAH validation of the test, the performance of the test has been evaluated at two cut-offs, a high sensitivity setting and a high specificity setting.
Table 1: Output showing quantitative output of the test
|
Spot ID |
RLU |
|
Ag1 |
60,119 |
|
Ag2 |
15,469 |
|
Ag3 |
52,118 |
|
Ag4 |
3,685 |
|
Ag5 |
29,121 |
|
Ag6 |
10,098 |
|
Ag7 |
54,434 |
|
Ag8 |
58,912 |
|
Ag9 |
22,551 |
|
Ag10 |
41,573 |
|
Ag11 |
65,232 |
|
Blank |
300 |
The timing of sampling after a tuberculin test (TT) significantly affects the sensitivity of detection of antibody. Samples taken approximately 5-30 days post-TT will benefit from the antibody ‘boosting’ effect due to an anamnestic response to PPD antigens in animals infected with bTB. Samples taken outside this ‘window’ (non-boosted) will have lower levels of antibody and the test will be less sensitive, although still more sensitive than the skin test (SICCT).
Performance of the test
The performance of the test, at these high sensitivity and high specificity settings, against defined populations of TB negative and positive animals in the United Kingdom, Ireland and worldwide has been elucidated. This work was performed as part of the requirements for test validation by the WOAH. If the samples are indicated as boosted within the tables below, this meant the sera that was analysed was collected within the 5-30 day window post tuberculin and thereby would be expected to have had an improved sensitivity, though it should be stated, that as it is anticipated this is when samples for the test would be taken in the field, the sensitivities displayed are appropriate to the expected performance of the test.
Table 2: Diagnostic specificity of the Enferplex Bovine TB antibody test using individual serum samples and bulk tank milk
|
bTB-free animals |
Number of animals |
Statistical Variable* |
High Sensitivity setting |
High Specificity setting |
|
Serum Non-boosted
|
4258 |
RSp CI |
98.4% 98.0 – 99.0 |
99.7% 99.5 – 99.8 |
|
Serum boosted |
339 |
RSp CI |
98.8% 97.0 - 99.5 |
99.1% 97.4 - 99.8 |
|
Bulk tank milk |
1792
|
RSp CI |
99.8% 99.4-99.9 |
99.9% 99.6-99.9 |
Table 3: Diagnostic sensitivity of the Enferplex Bovine TB antibody test using individual serum
|
bTB infection status POSITIVE by: |
Number of animals |
Statistical Variable* |
High Sensitivity setting |
High Specificity setting |
|
M. bovis culture Boosted |
214 |
DSn CI |
93.9% 89.9 - 96.4 |
93.9% 89.9 - 96.4 |
|
Visible Lesions Boosted |
1179 |
RSn CI |
96.1% 94.8 - 97.1 |
93.3% 93.3 – 95.9 |
|
SICCT test Boosted |
1949 |
RSn CI |
94.3% 93.2 - 95.2 |
91.9% 90.6 - 93.0 |
|
IFNg test Boosted |
1341 |
RSn CI |
90.0% 88.3 - 91.5 |
85.6% 83.6 - 87.4 |
Table 4: Diagnostic sensitivity of the Enferplex Bovine TB antibody test using bulk tank milk
|
Test method under evaluation |
Number of animals |
Statistical variable |
High Sensitivity |
High Specificity |
|
Relative sensitivity (Bulk tank milks contained R alone or R + IR) |
247 |
RSn CI |
77.7% 72.1-82.5 |
71.7% 65.4-76.9 |
Relationship between Number of Antigens recognised and the Presence of VL in animals seropositive by Enferplex Bovine TB
As with Johne’s disease, it is well accepted that in TB, as disease progresses, the production of antibody increases, and the strength of the cell mediated immune response wanes. We have analysed the correlation between antibodies, specifically the number of bTB antigens recognised by antibody-positive sera and the presence of VL at post-mortem examination. In total, 1933 SICCT test-positive UK and Irish animals were tested using the Enferplex Bovine TB antibody test at the high sensitivity setting.
The results were analysed with animals being classified as to how many antigens they recognised, e.g., 2 or more, 3 or more, etc., and this was correlated with the percentage with VL and as a percentage of the total VL animals in the population. The results showed that 91% of all Visible Lesions detected were in animals that had 5 or more antigens recognised by the Enferplex Bovine TB antibody test and that there was a good correlation between the number of antigens recognised by antibody and the presence of visible lesions in SICCT positive, IFNg positive or in-contact SICCT negative animals.
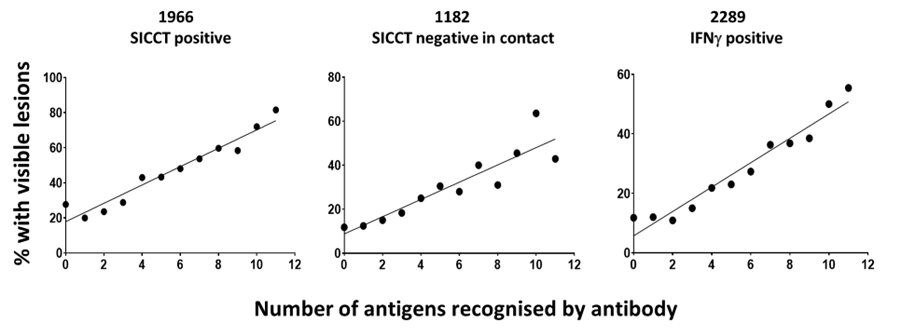
This shows that the Enferplex Bovine TB antibody test, as with Johne’s Disease, serological tests can aid in the identification of epidemiologically important bTB positive animals as those with lesions tend to be those which are excreting bacteria and are therefore infectious (Casal et al, 2014).
Use of bulk tank milk
The test is already validated by the World Organisation for Animal Health (WOAH) for use on individual blood samples, and it is now being evaluated as a bulk tank test. The validation studies are complete but are waiting to be approved by the relevant WOAH committees this summer.
Along with its potential ability to detect whether a herd is infected or not, the test potentially may also provide information about the dynamics of the disease within the herd (i.e. is the disease status improving or deteriorating) via reviewing the changes in the combined value of how many of the eleven antigens have tested positive and in the total amount of antibody detected.
What are the potential gains of a bulk tank milk test?
This is the first time a bulk tank milk test for bovine tuberculosis has been developed. Herd surveillance for bTB in dairy herds using bulk tank milk could revolutionise bTB surveillance and control because:
- Samples are easy to obtain, robust, reliable, and the test is inexpensive to perform.
- If it proves effective, it could reduce skin testing requirements for farms whether this be in the high-risk, edge or low risk areas of England or Wales or in Officially TB free Scotland.
- By allowing continuous monitoring of herds, it allows more rapid detection of disease introduction, making eradication easier.
- By allowing an insight into the infection dynamics of a herd, it could allow for more bespoke and suitable control measures to be taken.
As the exact extent of the value of bulk tank milk testing is not known at this stage, there is a clear requirement to perform a thorough field trial to assess the test. The difficulty in doing this is that there is a very limited time to perform such a study without risk to individual farms and the wider industry, as if the bulk tank milk test is officially validated by WOAH this summer, the UK Government would have to place restrictions on any herd testing positive. Prior to this though, Defra and the Devolved Authorities have stated that they will not act on any positive results and are under no obligation to do so.
Fortunately, with the combined help and goodwill of the Government and Devolved Administrations and many participants within the dairy industry, we have managed to set up this study in a very short period of time with over 5,000 dairy farms across Great Britain participating.
The details of the study are set out below.
The Study
We will be testing the bulk tank milk from approximately 5,000 dairy farms from across England, Scotland, and Wales with the Enferplex bTB Antibody test. Enrolled farms will have their bulk tank milk tested every 2 to 3 months, with a smaller subset of milk recorded herds tested monthly. The study will start in early February 2023 and end in September 2023 (or whenever the test is formally validated if WOAH approve the test).
Testing will be performed at the National Milk Laboratories in Glasgow using the payment testing samples so no new samples would need to be gathered from participating farms. The test results would be sent by NML to Scotland’s Rural College (SRUC) for analysis.
The SRUC statistical analyses will be anonymised before sending to Surefarm Ltd, Enfer Scientific Ltd and MV Diagnostics Ltd who would be responsible for collating and reporting the results to government, the farming community, and scientific journals.
The Government and Devolved Administrations have authorised this study to be performed.
Under law, Defra and the Devolved Authorities require the results of positive tests to be reported to them as a suspicion of notifiable disease. However, they have confirmed that there will be no repercussions for individual farms participating in the proposed study. Specifically, they will take no statutory actions if a bulk tank milk sample shows a positive Enferplex milk test result. The official TB status of any participating herds (whether free, suspended or withdrawn), and their normal TB statutory testing schedule will be unaffected by the study.
Specifically, the study will be focusing on:
- The test’s ability to detect disease in herds currently under restriction. While the sensitivity of the test has already been elucidated for the OIE submission, this sensitivity was based on a single time point and the repeatability of the test may allow for an enhanced sensitivity for detection of bTB at the herd level. (The sensitivity of the test, where a reactor contributing to the bulk tank was disclosed at TT2 and the sample was taken between TT1 and TT2 is 77.7%)
- How many herds currently designated OTF would be determined positive by the test.
- Understanding the value of monitoring the level of bulk tank milk seropositivity as an indicator of whether a herd is moving either towards disease freedom where the levels are falling, or, vice versa, where the levels are rising, that the current actions are failing to control disease where the herd is already under restriction, or where the herd is currently OTF, predicting that a herd will lose its OTF status.
- Understanding whether there is relationship between seropositivity and risk of bovine tuberculosis (bTB) breakdown.
Summary
We are very grateful to all who have helped in aiding this study to go ahead. We very much hope and expect that the results will demonstrate a clear benefit of bulk tank milk testing to the industry in fighting this devastating disease and we look forward to reporting the outcomes in the future.
References:
Casal et al (2014). Strategic use of serology for the diagnosis of bovine tuberculosis after intradermal skin testing. Vet Microbiol. 170: 342-351
Photography Credit @Jenny Wood Photography