
Take Home Messages
- Genome editing refers to the use of site directed nucleases (e.g. Zinc finger nuclease, TALENS, CRISPR/Cas9) to introduce targeted alterations into genomic DNA sequence.
- It offers a way to repair genetic defects, inactivate or knock-out undesired genes, or move beneficial alleles and haplotypes between breeds in the absence of linkage drag.
- Genome editing would synergistically complement, not replace, traditional breeding programs.
- It has been used to introduce useful genetic variants impacting disease resistance, product quality, adaptability, and welfare (e.g. polled) traits into cattle breeding programs.
- The regulatory oversight of genome editing in animals varies by jurisdiction; in 2018 the European Court of Justice ruled that organisms obtained by directed mutagenesis, including genome editing, will be regulated as genetically modified organisms (GMOs).
Abstract
Genome editing research in cattle to date has focused on disease resistance, production, product quality, and welfare traits. Modelling has revealed how editing could be used to introduce beneficial alleles into cattle breeds and maintain, or even accelerate, the rate of genetic gain accomplished by conventional breeding programs. As with earlier genetic engineering approaches, whether breeders will be able to employ the breeding method in cattle genetic improvement programs will depend upon the regulatory framework and governance of genome editing for food animals.
Introduction
The previous generation of genetic engineering tools, resulting in the first transgenic livestock over 30 years ago, was limited to the insertion of foreign DNA into the genome. As integration was random, there was no way of predicting all of the possible effects that introducing the transgene would have on the animal as the epigenetic environment varies among different regions of the genome. It also meant that each genetically engineered founder animal had the gene inserted into a different location in the genome. To date, there is only a single approved genetically engineered animal for food purposes globally, the fast-growing AquAdvantage Atlantic salmon.
Genome editing presents an approach to introduce targeted modifications into existing genes and regulatory elements within a breed or species, without necessarily introducing foreign DNA, potentially avoiding concerns regarding transgenesis. It offers a new opportunity to accelerate the rate of genetic gain in livestock by precisely introducing useful extant genetic variants into structured livestock breeding programs. These variants may repair genetic defects, inactivate or knock-out undesired genes, or involve the movement of beneficial alleles and haplotypes between breeds in the absence of unwanted genetics from the donor breed.
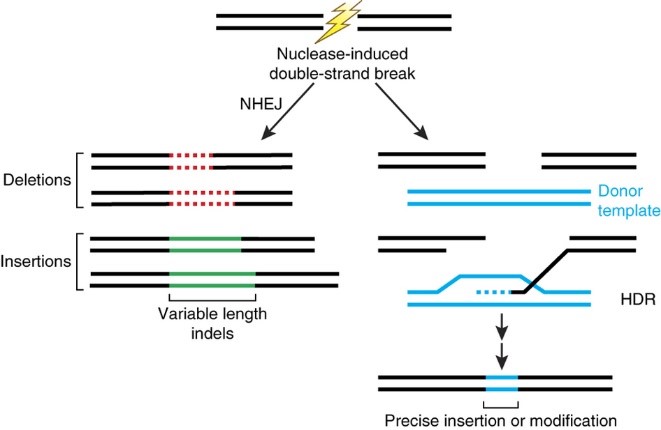
Genome or gene editing refers to the use of site-directed nucleases (e.g. Zinc finger nuclease, TALENS, CRISPR/Cas9) to precisely introduce a double stranded break (DSB) at a predetermined location in the genome. The cell can repair that DSB break in one of two ways – nonhomologous end joining (NHEJ) or homologous-directed repair (HDR) using a nucleic acid donor template that includes the sequences homologous to either side of the double-strand break. The outcomes of these repair processes result in random mutations, or precise gene edits, respectively (see Figure 1).
Figure 1: Nuclease-induced double-strand breaks can be repaired by nonhomologous end joining (NHEJ) or homology-directed repair (HDR) pathways. Imprecise NHEJ-mediated repair can produce insertion and/or deletion mutations (indels) of variable length at the site of the double-strand break. HDR-mediated repair can introduce precise point mutations or insertions from a single-stranded or double-stranded nucleic acid donor template
Genome editing research in cattle to date has focused primarily on monogenic (single gene) traits like disease resistance (e.g. tuberculosis), production (e.g. myostatin knockout), generation of sex-ratio skews (e.g. increase proportion of male calves), elimination of allergens (e.g. betalactoglobulin knockout), and welfare traits (Table 1). In the absence of editing, livestock producers may be resistant to the introduction of a useful new allele that is associated with sires of inferior genetic merit, or sires from another breed. For example, the polled allele which results in hornlessness is associated with dairy sires of lower genetic merit, and bulls from several beef breeds. Using gene editing, this allele has been introduced into dairy genetics in a proof of concept experiment. If this allele could be edited into the genome of a number of elite dairy sires, then artificial insemination could be used to disseminate that dominant polled allele through the dairy cattle population, and eliminate the need for horn removal which is an animal welfare concern.
Table 1: Examples of proposed and potential targets for genome editing in cattle
|
Target |
Targeted Trait/Goal |
|
Myostatin (MSTN) gene knockout |
Increased lean muscle yield |
|
Intraspecies POLLED allele substitution |
No horns/welfare trait |
|
Intraspecies SLICK allele knockout |
Heat tolerance |
|
Diluted coat color |
Heat tolerance |
|
Beta-lactoglobulin gene knockout |
Elimination of milk allergen |
|
CALPAIN & CAPASTATIN allele substitution |
Improved meat tenderness |
|
Omega-3 (Fat-1) transgene insertion |
Increased omega-3 fatty acids |
|
Prion protein (PRNP) knockout |
Disease Resistance (Bovine spongiform encephalopathy (BSE) or “Mad Cow” Disease) |
|
Insertion of lysostaphin transgene |
Disease Resistance (Mastitis) |
|
CD18 gene edit |
Disease Resistance (Bovine Respiratory Disease) |
|
Insertion of SP110, NRAMP1 gene |
Disease Resistance (Tuberculosis) |
|
Intraspecies SRY gene duplication |
Increased proportion of male offspring |
|
NANOS gene knockout |
Infertile males (for surrogate sire) |
One could potentially envision editing several alleles for different traits – such as known fertility impairing haplotypes, polled, and to correct known Mendelian genetic defects that affect cattle, all while using conventional selection methods to keep making genetic progress towards a given selection objective. Although single gene traits present good targets for genome editing and can have tangible animal health, environmental and economic outcomes, nearly all economically important livestock traits are complex traits controlled by many genes. These include milk yield and composition, carcass yield, composition and quality, feed conversion, feed efficiency, growth rate, wool yield and quality, fertility, egg yield, and disease resistance. Some individual genes can have large effects on polygenic traits: for example, mutations in the myostatin gene that result in ‘double muscle’ phenotypes greatly contribute to the polygenic trait of carcass yield. Other alleles with more modest effects on polygenic traits could still be good targets for gene editing, particularly with the inevitable improvements in gene editing that will meet the coming decades.
Genome editing conceptually offers an approach to translate the thousands of single nucleotide polymorphisms (SNP) markers discovered through livestock sequencing projects, the information obtained from numerous genome wide association studies, and the discovery of causative SNPs (Quantitative Trait Nucleotides; QTNs) into useful genetic variation for use in animal breeding programs. One modeling study reported that combining gene editing with traditional genomic selection could improve the response to selection four-fold after 20 generations. It is worth noting, however, that this study modeled editing a complex polygenic trait that had 10,000 known QTN. In reality, breeders do not currently have a comprehensive understanding of which edits would be impactful on quantitative traits, i.e. those controlled by many genes. It is unlikely that all of the genes affecting such traits are known, nor is it typically evident which edits might be the most desirable for these genes (i.e. what is the sequence of the desirable allele). It is likely that, at least in the short term, editing will focus on large effect loci and known targets to correct genetic defects or decrease disease susceptibility, and conventional selection will continue to make progress in selecting for all of the many small effect loci that influence the complex traits that contribute to the breeding objective. In other words, editing will complement, not replace, conventional breeding programs.
Intersection with Conventional Breeding
To become an important driver of genetic change, genome editing methods must seamlessly integrate with conventional animal breeding programs. That means that they must reliably function to germline-edit animals that are selected to be the next generation of parents. Edits can be introduced through gene editing of somatic cells followed by somatic cell nuclear transfer (SCNT) cloning (e.g. Dolly), or introduction of the gene editing reagents into zygotes (early-stage one cell embryo) of the next generation of selection candidates (see Figure 2).
Figure 2: Steps for producing gene edited livestock through somatic cell nuclear transfer (SCNT) cloning (left) or zygote editing (right). Schematic showing the typical steps involved to produce homozygous, non-mosaic livestock by either SCNT cloning of gene edited and screened somatic cells or cytoplasmic injection (CPI)/electroporation (EP) of zygotes with gene editing components
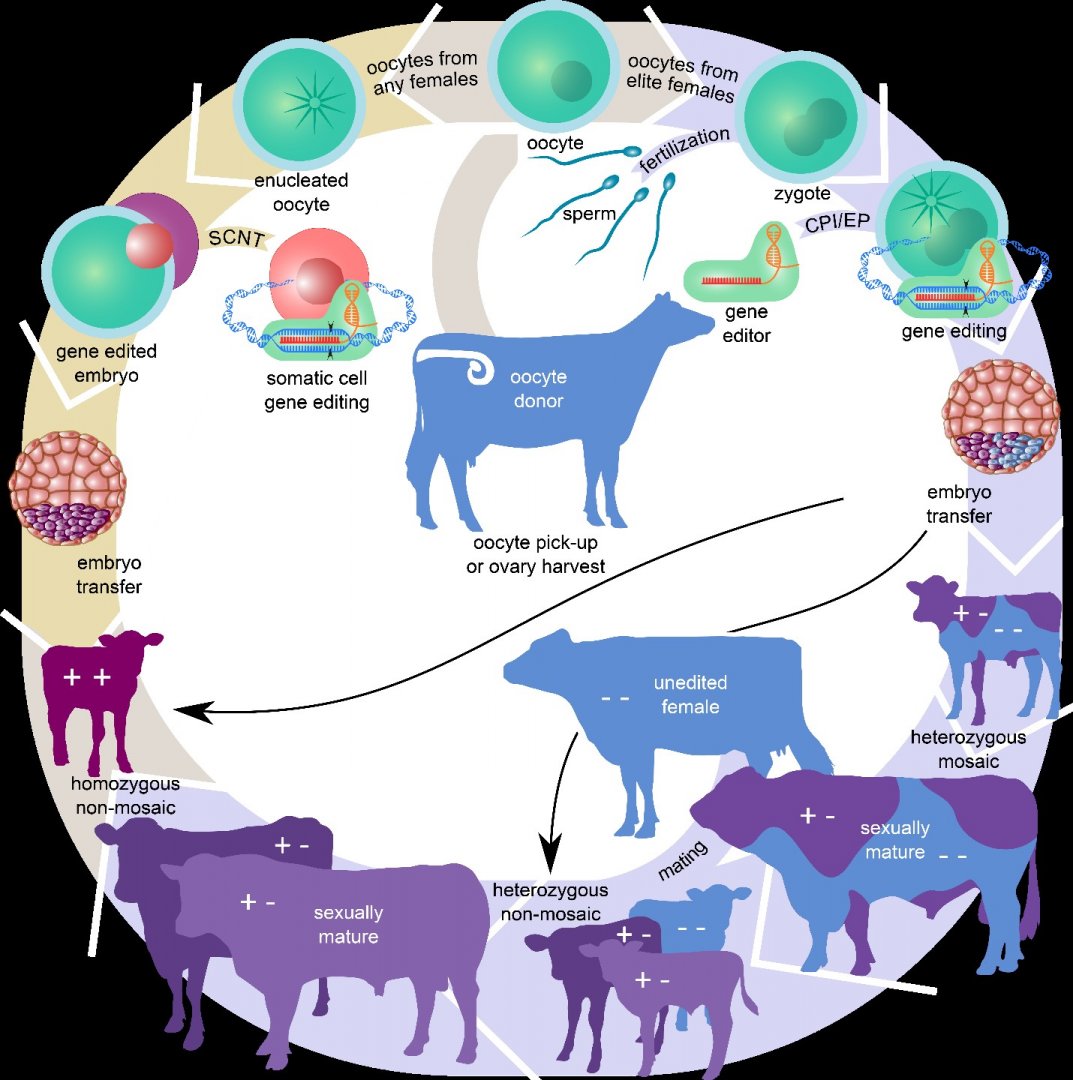
Image from Bishop and Van Eenennaam (2020) Journal Experimental Biology 223: jeb207159 .
To date, SCNT cloning has been the primary method to deliver nuclease-mediated genetic changes into livestock. The advantage of SCNT is that the gene edited cell line can be genotyped and/or screened prior to transfer into the enucleated oocyte to ensure that the desired edits, and no donor template integrations, have occurred. The disadvantage is that there are well-documented drawbacks and inefficiencies associated with cloning including early embryonic losses, and birth defects. Additionally, the EU parliament voted to ban the cloning of farm animals in 2015.
Direct editing of zygotes is advantageous since it is modifying the next generation, but the disadvantage is that not all embryos will have the desired edit, and often embryos are mosaic – meaning some cells are edited and some are not. However, on average fewer embryos are required to gene edit a pig, for example, using this approach as compared to SCNT due to the inefficiencies associated with cloning. Knockouts using NHEJ have been achieved through editing of zygotes from a number of livestock species, and can be obtained with relatively high frequency, with some reports of 100% efficiency. Targeted gene knock-ins using a donor repair template and HDR have proven more challenging, although my laboratory reported the birth of a zygote-mediated genome edited SRY knockin bull calf in 2020. The calf is predicted to produce 75% male offspring.
Regulations
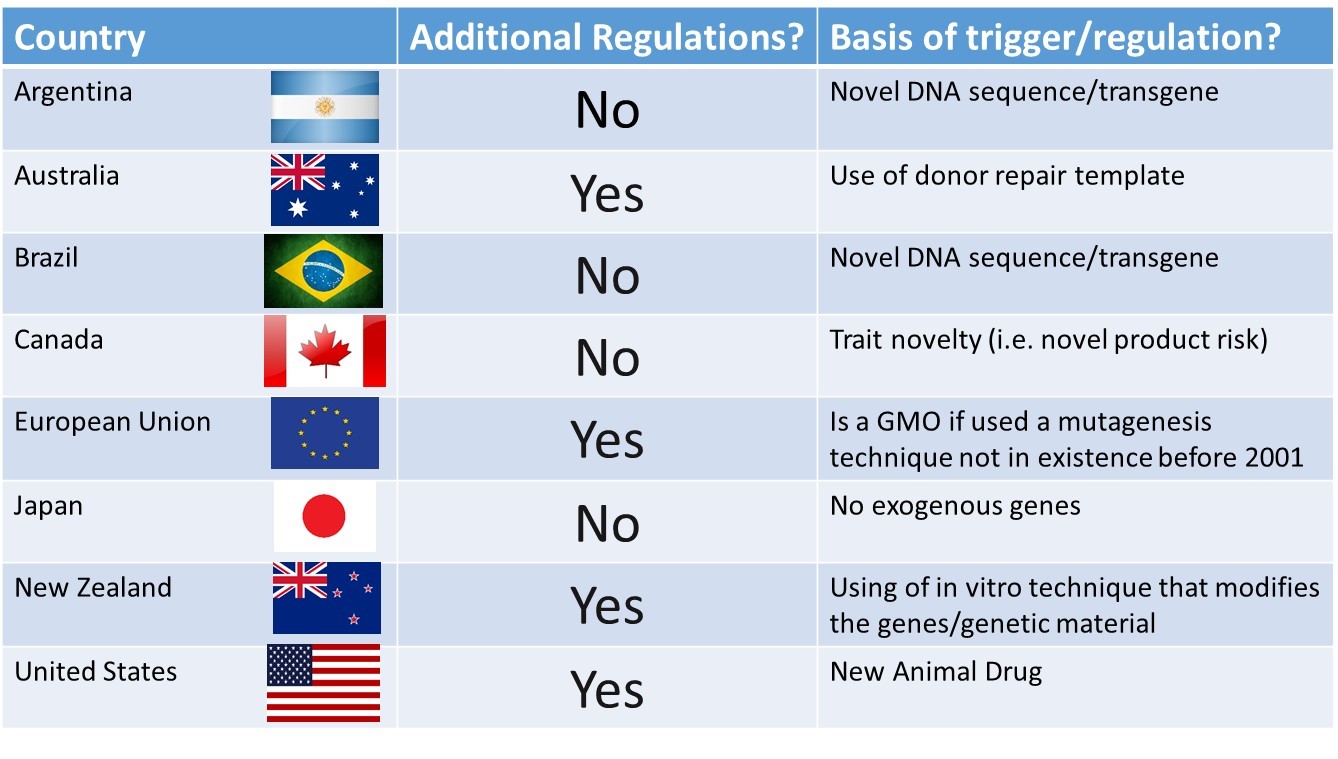
As with earlier genetic engineering approaches, whether breeders will be able to employ genome editing in cattle genetic improvement programs will very much depend upon global decisions around the regulatory framework and governance of genome editing for food animals. Currently, the global regulatory status of genome edited livestock is uncertain, and is the primary concern for further investment and development of gene edited animals. Editing that does not introduce novel recombinant DNA sequences would not be treated differently to conventional breeding in some countries e.g., Argentina, Brazil and Australia; whereas in the US all intentional genomic alterations in animals are going to be regulated as veterinary drugs. Similarly, in 2018 the European Court of Justice ruled that organisms obtained by directed mutagenesis, including genome editing, will be regulated as genetically modified organisms (GMOs). Additionally, in the European Union, animal cloning is currently prohibited which would also preclude SCNT-enabled editing approaches. Conversely, in Canada traditional mutagenesis is not regulated unless it produces a novel trait. In that country it is the novelty of product that triggers regulation, not the method or process used to produce that novel trait. And finally, New Zealand plans to regulate all genome edited plants and animals, irrespective of the nature of the edit, as GMOs.
From a risk perspective, it does not make a lot of sense to have a different set of regulations for a genome edited calf carrying a naturally-occurring genetic variant such as the polled allele (free of any foreign or ‘transgenic’ DNA), as compared to a calf that inherited that same naturally-occurring allele from its parents. The regulatory situation in selected countries is summarized in Figure 3. Many countries have made regulatory decisions about genome editing in livestock in the absence of engagement with the scientific community, industry, stakeholders or publics. In particular, the judgement of the European Court of Justice to subject gene edited organisms to GMO regulations, but to exempt organisms produced using the older less precise process of mutagenesis breeding defied risk proportionality and reasonableness, to the dismay of many European scientists. It remains to be seen whether animal breeding companies can successfully overcome the technical and regulatory challenges that must be faced to employ gene editing for the genetic improvement of commercial livestock.
Figure 3: Breakdown by country of whether a gene-edited knock-in via HDR of donor template of a naturally-occurring allele (e.g. polled) in livestock would be subject to additional regulations.
Conclusions
Significant improvements in the efficiency of milk and beef production have historically been accomplished through conventional breeding of superior individuals with an eye towards specific breeding objectives. Genome editing is a tool that is well suited for modifying qualitative, single gene traits at comparatively rapid rates in the absence of linkage drag, and could be used in conjunction with conventional selection approaches to address issues such as disease resistance and improved welfare traits. Animal breeders need regulatory certainty regarding genome editing. If editing is used to introduce alterations that are no different from those that could have been obtained using conventional breeding, risk proportionality suggests it should not trigger additional layers of regulatory scrutiny and expense. Regulations should be proportionate to any novel risks inherent in the product, and not the process used to produce that product. Regulating genome edited livestock as GMOs based on the use of genome editing techniques, as has been proposed in the EU, USA and New Zealand, will effectively forestall the application of genome editing in animal agriculture in these countries for the foreseeable future. This will put them at a disadvantage when it comes to incorporating genome editing into livestock breeding programs, as compared to countries (e.g. Brazil, Argentina, Canada) where novel product risk-based regulatory approaches are being implemented.
Acknowledgements
The author acknowledges funding support from the National Institute of Food and Agriculture and the Biotechnology Risk Assessment Grant (BRAG) program, U.S. Department of Agriculture, under award numbers 2017-33522-27097, 2018-67030- 28360, 2020-67015-31536, and 2020- 70410-32899