Abstract
A number of breeding methods have been employed in genetic improvement programs to achieve genetic gains including artificial insemination, embryo transfer, crossbreeding, and more recently genomic selection. These technologies are limited by the fact that identifying desirable new genetic variation is a matter of chance; selection may inadvertently leave behind favorable variants that existed in founder populations, and breeders may unintentionally propagate deleterious mutations because they are closely linked to advantageous variants. Genome editing offers a way to avoid these difficulties by precisely introducing desirable genetic variation into livestock breeding programs. Both intra- and interspecies allele substitutions and gene knock-ins have been accomplished with genome editing tools, targeting a number of important traits. The regulatory status of such animals is unclear as the definition of a regulated article is not consistent among different regulatory agencies and organizations. In the absence of a harmonized global regulatory approach to gene editing, it will be difficult for breeders to effectively use this technology to introduce precise genetic variations into breeding programs.
Genome Editing
Genome or gene editing refers to the use of site-directed nucleases to precisely introduce a double stranded break at a predetermined location in the genome. The cell can repair that DSB break in one of two ways – nonhomologous end joining (NHEJ) or homologous-directed repair (HDR) using a nucleic acid template that includes the sequences homologous to either side of the double-strand break. The outcomes of these repair processes result in random mutations or precise gene edits, respectively (Figure 1).
Figure 1: Nuclease-induced double-strand breaks can be repaired by nonhomologous end joining (NHEJ) or homology-directed repair (HDR) pathways. Imprecise NHEJ-mediated repair can produce insertion and/or deletion mutations of variable length at the site of the double-strand break. HDR-mediated repair can introduce precise point mutations or insertions from a single-stranded or double-stranded DNA donor template.
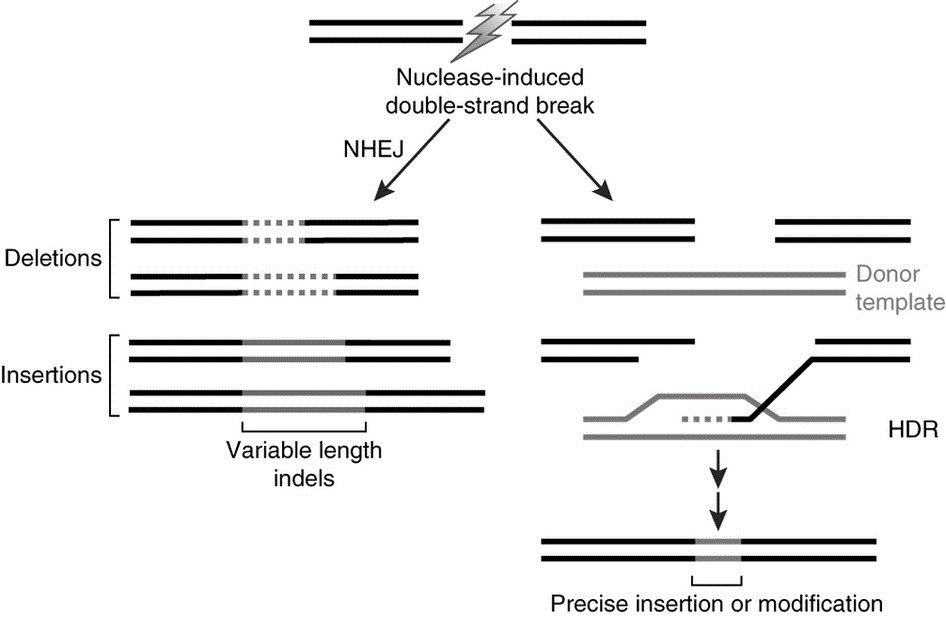
As the name ‘gene editing’ suggests, HDR can be employed to precisely add, delete, or replace letters in the genetic code at the location of the break by providing the appropriate template nucleic acid. In the case of NHEJ, although the location of the cut site is very precise, the exact change that occurs when the DNA is repaired is random so a number of different outcomes representing minor sequence insertions (ins) or deletions (del), termed indels, are possible.
Gene editing has many potential applications. For example, it can be used to correct diseases and disorders that have a genetic basis by altering the error that resulted in the disease phenotype. It could also be used to change a less desirable allele of a gene to a more desirable allele without the need to introgress (repeatedly backcross) or bring in that allele through outcrossing with an animal that carries the desirable allele. Genome editing technologies enable breeders to efficiently turn off a gene through NHEJ or precisely introduce specific allelic variants. This introduction could be as simple as a single base pair change or could conceptually involve entire genes or transgenes, as dictated by the HDR template nucleic acid sequence, that breeders would like to introduce into their target population using editing.
How might gene editing be used in animal breeding?
In the last five years, gene editing has been used to target traits associated with product yield, animal health and welfare in several species as summarized in Table 1 (below). Unsurprisingly, the goals of genetic improvement programs do not change with new breeding tools; breeders tend to employ whichever breeding method(s) most effectively achieves progress towards their breeding objective(s). Gene editing has the potential to synergistically complement traditional breeding programs, rather than replace or radically disrupt them.
Table 1: Examples of successful gene edited agricultural applications in food animal species. Knock out=inactivation of gene function
| Species | Target | Targeted Trait/Goal |
| Cattle | Intraspecies POLLED allele substitution | No horns |
| Myostatin gene knockout | Increased muscle yield | |
| Beta-lactoglobulin gene knockout | Elimination of milk allergen | |
| Insertion of lysostaphin transgene | Disease resistance | |
| Insertion of lysozyme transgene | Disease resistance | |
| Insertion of SP110 transgene | Resistance to tuberculosis | |
| Chicken | Ovalbumin gene knockout | Elimination of ovalbumin in egg |
| Insertion of Immunoglobulin heavy chain locus | Germline gene editing | |
| Goat | Myostatin gene knockout | Increased muscle growth |
| Prion protein gene knockout | Elimination of prion protein | |
| Beta-lactoglobulin gene knockout | Elimination of milk allergen | |
| Pig | CD163 gene knockout | PRRS Virus Resistance |
| Interspecies RELA allele substitution | African Swine Fever Resistance | |
| Myostatin gene knockout | Increased muscle yield | |
| Sheep | Myostatin gene knockout | Increased muscle yield |
How might gene editing intersect with conventional breeding?
Data coming out of some of the large-scale genomic and sequencing projects are revealing situations in which the sequence of one naturally occurring allele results in superior performance to that observed when animals inherit the alternative allele of that gene. It is envisioned that it might be possible to edit an animal’s genome to the superior allele, and to do that at several genomic locations, or for several different genes. Simultaneous targeting of different genes has allowed bi-allelic modification of up to three genes at the same time. The advantage of gene editing over conventional selection to move these naturally occurring alleles from one animal to another is that favorable alleles rarely all occur in one single individual and editing offers the opportunity to increase the frequency of desirable alleles in an individual or a breed more rapidly than could be achieved through conventional breeding, and in the absence of undesirable linkage drag.
One could potentially envision editing several alleles for different traits of importance to beef cattle production systems and product quality – such as known fertility impairing haplotypes, polled, myostatin, calpain, calpastatin, steatoyl-CoA desaturase, and to correct known Mendelian genetic defects that affect cattle – all while using conventional selection methods to keep making genetic progress towards a given selection objective. Gene editing offers an approach to translate the thousands of SNP markers discovered through livestock sequencing projects, the information obtained from numerous genome wide association studies, and the discovery of causative SNPs(Quantitative Trait Nucleotides; QTNs) into useful genetic variation for use in animal breeding programs. One simulation study reported that combining gene editing with traditional genomic selection could improve the response to selection four-fold after 20 generations. In other words, editing will complement, not replace, conventional breeding programs.
It should be remembered that complex traits are typically impacted by many different genes. It is unlikely that all of the genes impacting such traits are known, nor is it typically evident which might be the desirable molecular edits for these genes (i.e. what is the sequence of the desirable allele). It is likely that editing will be focused on large effect loci and known targets to correct genetic defects or decrease disease susceptibility, and conventional selection will continue to make progress in selecting for all of the many small effect loci that impact the complex traits that contribute to the breeding objective.
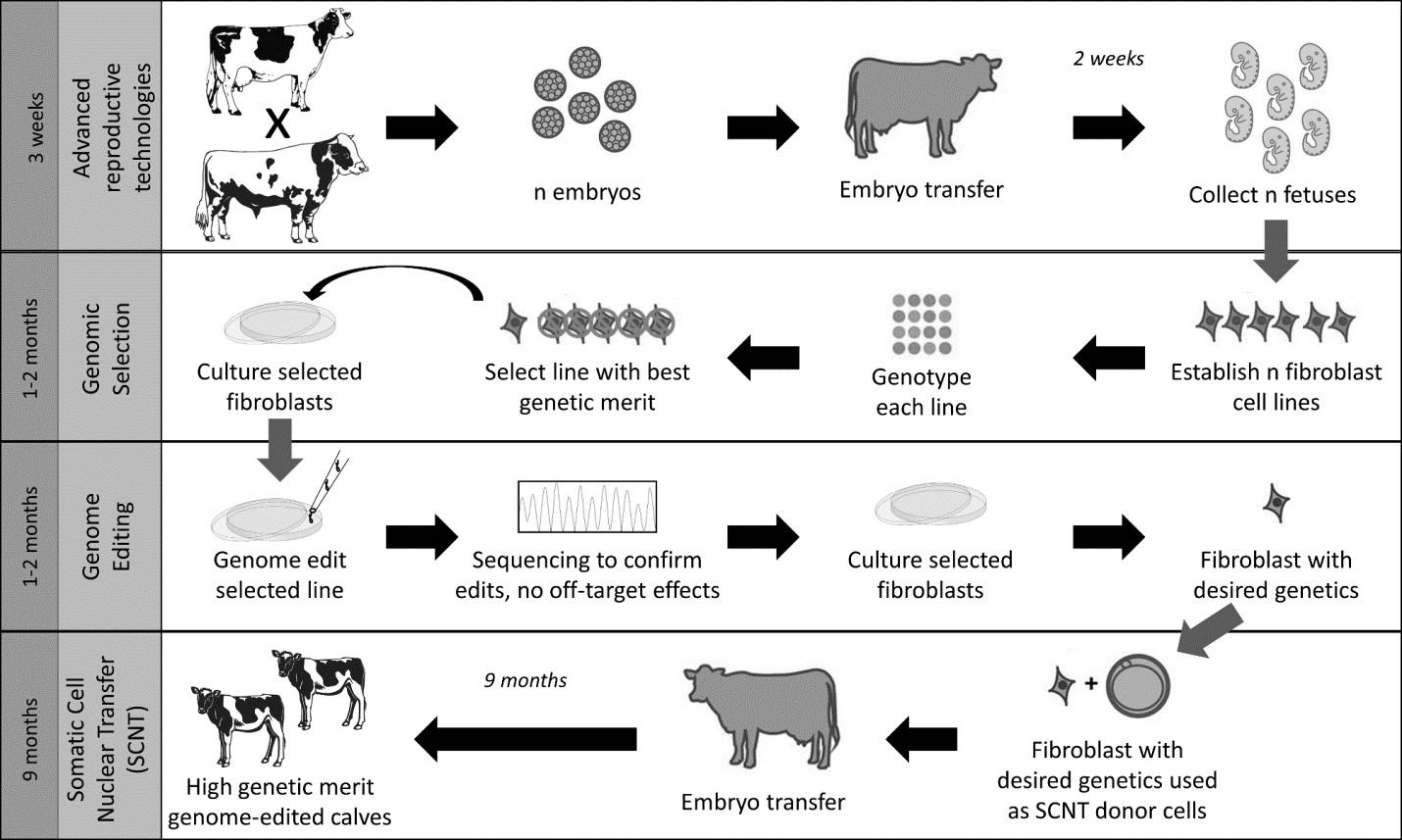
To become an important driver of genetic change, gene editing methods must seamlessly integrate with conventional animal breeding programs. That means that they must reliably function to germline-edit animals that are selected to be the next generation of parents. Edits can be introduced through gene editing of somatic cells followed by somatic cell nuclear transfer cloning, or injection of the gene editing reagents into the cytoplasm of single cell zygotes of the next generation. A potential approach to incorporate genome editing in current breeding programs through the use of advanced reproductive technologies are outlined in Figure 2.
Figure 2: Production of high genetic merit calves using a range of biotechnologies and showing where gene editing might fit into the process. Figure from Van Eenennaam, A.L. 2017. Genetic Modification of Food Animals. Current Opinion in Biotechnology 44: 27–34.
Will gene editing be regulated?
Globally, governments and regulators are currently deliberating about how, or even if, gene-edited animals should be regulated. It is currently unclear whether gene editing is going to be regulated as ‘genetic engineering’ (GE), and the answer to that question will likely determine how widely this technique is used in animal breeding programs. Genetic engineering has been mired in a worldwide furor for the past quarter century, and to date there are no commercialized GE food animals on the market anywhere in the world. If gene editing gets conflated with GE, then it is unlikely that this technique will be available to animal breeders.
The Codex Alimentarius (Codex), or ‘Food Code,’ was established by FAO and WHO to develop harmonised international food standards, which protect consumer health and promote fair practices in food trade. In 2008 the Codex developed the science based ‘Guideline for the Conduct of Food Safety Assessment of Foods Derived from Recombinant-DNA Animals (GL68-2008)’ which provides internationally-recognized recommen dations for assessing the nutrition and safety of food from GE animals. In that document, a ‘Recombinant-DNA Animal’ is defined as an animal in which the genetic material has been changed through in vitro nucleic acid techniques, including rDNA and direct injection of nucleic acid into cells or organelles.
The Cartagena Protocol on Biosafety (CPB) is an international agreement which aims to ensure the safe handling, transport and use of any living modified organism. The CPB defines ‘Living modified organism’ to mean any living organism that possesses a novel combination of genetic material obtained through the use of modern biotechnology, and specifically excludes techniques used in traditional breeding and selection. Likewise, the EU definition of a genetically engineered organism included in Directive 2001/18/EC encompasses an ‘organism, with the exception of human beings, in which the genetic material has been altered in a way that does not occur naturally by mating and/or natural recombination.’ And finally, the United States FDA defines ‘genetically engineered (GE) animals’ as those animals modified by recombinant DNA (rDNA) techniques, including the entire lineage of animals that contain the modification. The rDNA construct in the GE animal, not the animal itself, is considered a new animal drug and thus is a regulated article under the new animal drug provisions of the Federal Food Drug and Cosmetics Act. All GE animals are captured under these provisions, regardless of their intended use.
It is clear that many gene editing applications will likely result in animals carrying desirable alleles or sequences that originated in other breeds or individuals from within that species (e.g. hornless Holsteins were edited to carry the Celtic polled allele found in breeds like Angus). As such, there will be no rDNA construct in the animal or novel combination of genetic material. The genetic material will also not be altered in a way that could not be achieved by mating or techniques used in traditional breeding and selection. As such, at the current time it is unclear whether gene editing will be formally regulated as is the case with animals containing transgenic rDNA constructs (e.g. AquAdvantage salmon) that were the products of ‘traditional’ genetic engineering.
Gene editing does not necessarily introduce any foreign genetic DNA or ‘transgenic sequences’ into the genome, and many of the changes produced would be indistinguishable from naturally-occurring alleles and genetic variations. As such, many applications will not fit the classical definition of genetic engineering. For example, many edits are likely to alter alleles of a given gene using a template nucleic acid dictated by the sequence of a naturally-occurring allele from the same species. As such, there will be no novel DNA sequence present in the genome of the edited animal, and likewise no novel phenotype associated with that sequence. It is not evident what unique risks might be associated with an animal that is carrying such an allele given the exact same sequence and resulting phenotype would be observed in the closely related breed from which the allele sequence was derived.
It is possible that editing might introduce double stranded breaks at locations other than the target locus,and thereby induce alterations elsewhere in the genome. Such ‘off target events’ are analogous to the naturally-occurring spontaneous mutations that produce the genetic variation that drives evolution. Complete sequencing of two Holstein calves that were edited to carry the polled allele revealed no unintended introgression of the polled allele, nor any insertion-deletions (indels) ascribable to off-target DNA breaks. They were essentially Holsteins that were homozygous for the Celtic polled allele at the polled gene locus.
It is no coincidence that there have been a slew of recent policy papers from normally low-profile public sector plant and animal breeders and academicians from around the world discussing the need for regulation of genome editing to be science-based, proportional to risk, product focused, and fit for purpose. Many fear that this technology will suffer the same fate as genetic engineering, and will therefore not be available to the animal breeding community. Most recently the US National Academy of Sciences concluded that the distinction between conventional breeding and genetic engineering is becoming less obvious. They reasoned that conventionally bred varieties are associated with the same benefits and risks as genetically engineered varieties. They further concluded that a process-based regulatory approach is becoming less and less technically defensible as the old approaches to genetic engineering become less novel and as emerging processes – such as gene editing – fail to fit current regulatory categories of genetic engineering. They recommended a tiered regulatory approach focused on intended and unintended novel characteristics of the end product resulting from the breeding methods that may present potential hazards, rather than focusing regulation on the process or breeding method by which that genetic change was achieved.
It is likely that gene edited animals will be regulated on a case-by-case basis depending upon the novelty of the edited DNA sequence and the resulting attributes or phenotypes displayed by the animal. There is a need to ensure that the extent of regulatory oversight is proportional to the unique risks, if any, associated with the novel phenotypes, and weighed against the resultant benefits. This question is of course important from the point of view of technology development, innovation and international trade. Given there is currently not a single genetically engineered animal being sold for food anywhere in the world, animal breeders are perhaps the group most aware of the chilling impact that regulatory gridlock can have on the deployment of potentially valuable breeding techniques. While regulation to ensure the safety of new technologies is necessary, in a world facing burgeoning animal protein demands, overregulation is an indulgence that global food security can ill afford.
Acknowledgements
This work was supported by Biotechnology Risk Assessment Grant Program Competitive Grant no. 2015-33522-24106 and Agriculture and Food Research Initiative Competitive Grant no. 2015-67015-23316 from the National Institute of Food and Agriculture/U.S. Department of Agriculture.